In the European Union, Nuwiq® is indicated for the treatment and prophylaxis of bleeding in patients with haemophilia A (congenital factor VIII deficiency)5
Nuwiq® can be used for all age groups5
Nuwiq® was designed to closely mimic native FVIII1
Nuwiq® has no chemical modifications or fusion with other proteins2
No animal or human-derived materials are added during the manufacturing process or to the final medicinal product1

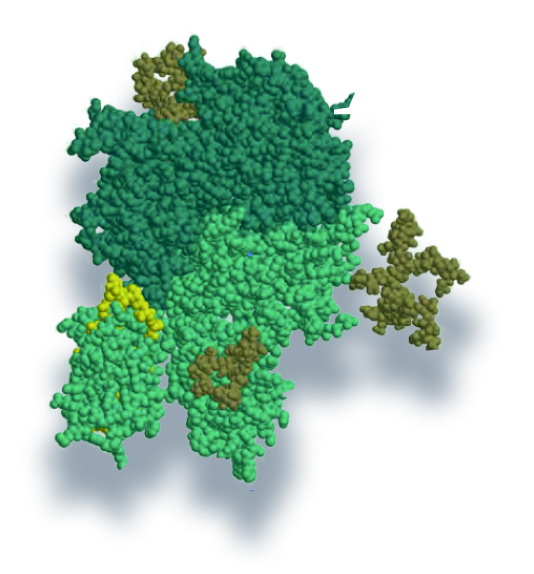



Human cell lines can accurately replicate human biochemical protein modifications without producing1,3 potentially antigenic epitopes
When proteins are produced in non-human
cells, such as baby hamster kidney (BHK) or
Chinese hamster ovary (CHO) they undergo
changes that create epitopes not found in
human proteins4
These non-human epitopes might trigger an
immune response in humans, producing
antibodies (inhibitors)4
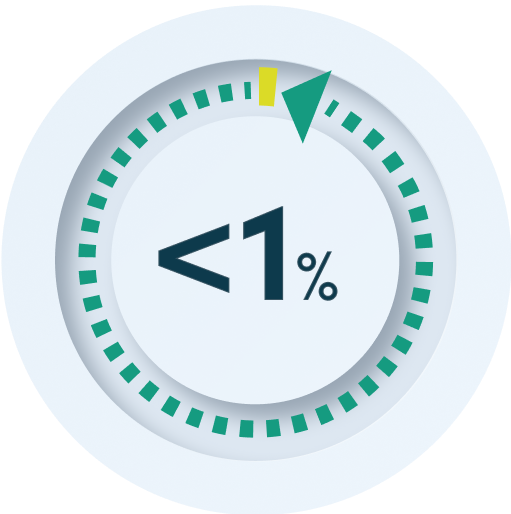
Nuwiq® has been shown to have high VWF-
binding affinity, and less than 1% unbound FVIII1

Human cell lines can accurately replicate human biochemical protein modifications without producing1,3 potentially antigenic epitopes
When proteins are produced in non-human
cells, such as baby hamster kidney (BHK) or
Chinese hamster ovary (CHO) they undergo
changes that create epitopes not found in
human proteins4
These non-human epitopes might trigger an
immune response in humans, producing
antibodies (inhibitors)4


Nuwiq® has been shown to have high VWF-
binding affinity, and less than 1% unbound FVIII1
In clinical trials and real-world clinical practice, Nuwiq® has proven all-round efficacy for prophylaxis, treatment of bleeds and surgery in haemophilia A patients of all ages2,6

Nuwiq® is a recombinant FVIII produced in a human cell line2
Valentino LA et al. Haemophilia 2014; 20(Suppl.1):1-9;
Lissitchkov T et al. Ther Adv Hematol 2019; 26;10:2040620719858471;
Kessler CM et al. Haemophilia 2015; 21(Suppl.1):1-12;
Kannicht C, et al. Thromb Res 2013; 131:78–88;
European Medicines Agency. Nuwiq Summary of Product Characteristics. Updated March 2023;
Mathias M et al. Haemophilia 2023; 29:1005-12.